



The CHIANG LAB
- Publications
- …
- Publications



The CHIANG LAB
- Publications
- …
- Publications

Metal coordinations
Inorganic Chemistry by EPR
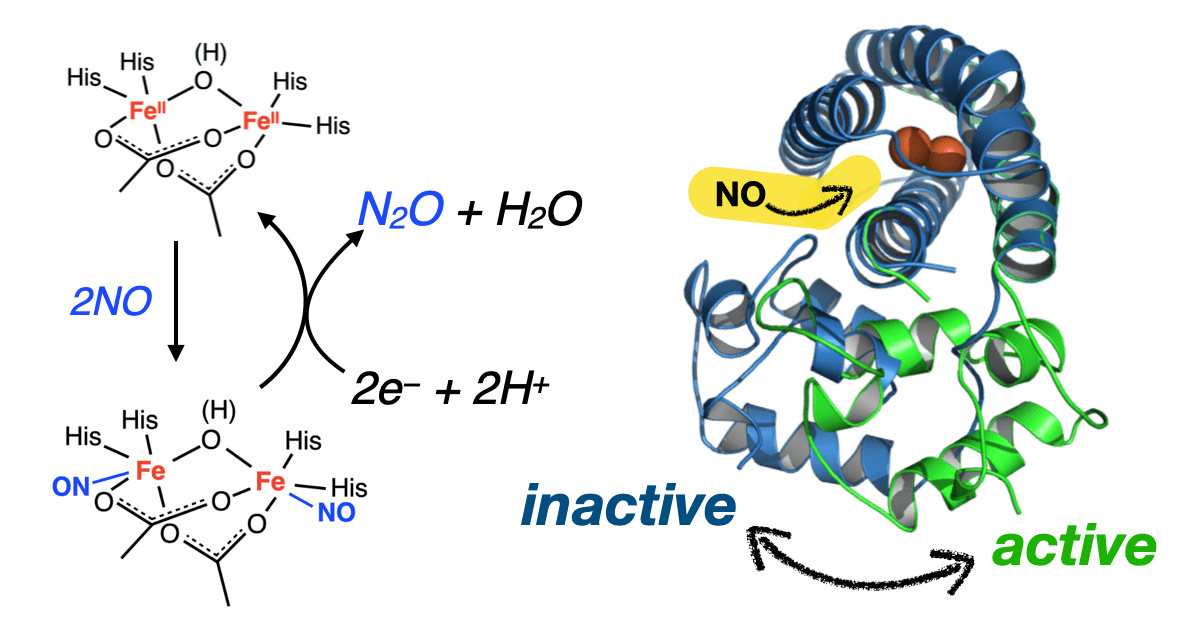
The YtfE protein catalyzes the reduction of NO to N2O, protecting iron-sulfur clusters from nitrosylation. The structure of YtfE has a two-domain architecture, with a diiron-containing C-terminal domain linked to an N-terminal domain, in which the function of the latter is enigmatic. Here, by using electron spin resonance (ESR) spectroscopy, we show that YtfE exists in two conformational states, one of which has not been reported. Under high osmotic stress, YtfE adopts a homogeneous conformation (C state) similar to the known crystal structure. In a regular buffer, the N-terminal domain switches between the C state and a previously unidentified conformation (C′ state), the latter of which has more space at the domain interface to allow the trafficking of NO molecules and thus is proposed to be a functionally active state. The conformational switch between the C and C′ states is pivotal for facilitating NO access to the diiron core.

Repair of Iron Centers Protein YtfE
ESR and Pulsed ESEEM results show that each iron of the oxo- bridged Fe(II)–Fe(III) diiron core is coordinatively unsaturated with each iron bound to two bridging carboxylates and two terminal histidines in addition to an oxo-bridge.
This result suggests that, in addi- tion to any repair of iron centers (RIC) activity, YtfE acts as an NO-trapping scavenger to promote the NO to N2O transformation under low NO flux, which precedes nitrosative stress.
Chemistry − A European Journal, 22 (2016) 9768-9776

EPR as a Tool To Investigate the Transition Metal Chemistry

EPR spectrum of Sn0.96Mn0.04HPO3 obtained at temperature 4.2 K
Chiang Lab@NTHU



